27 Feb Where is Chlorine Usually Used?
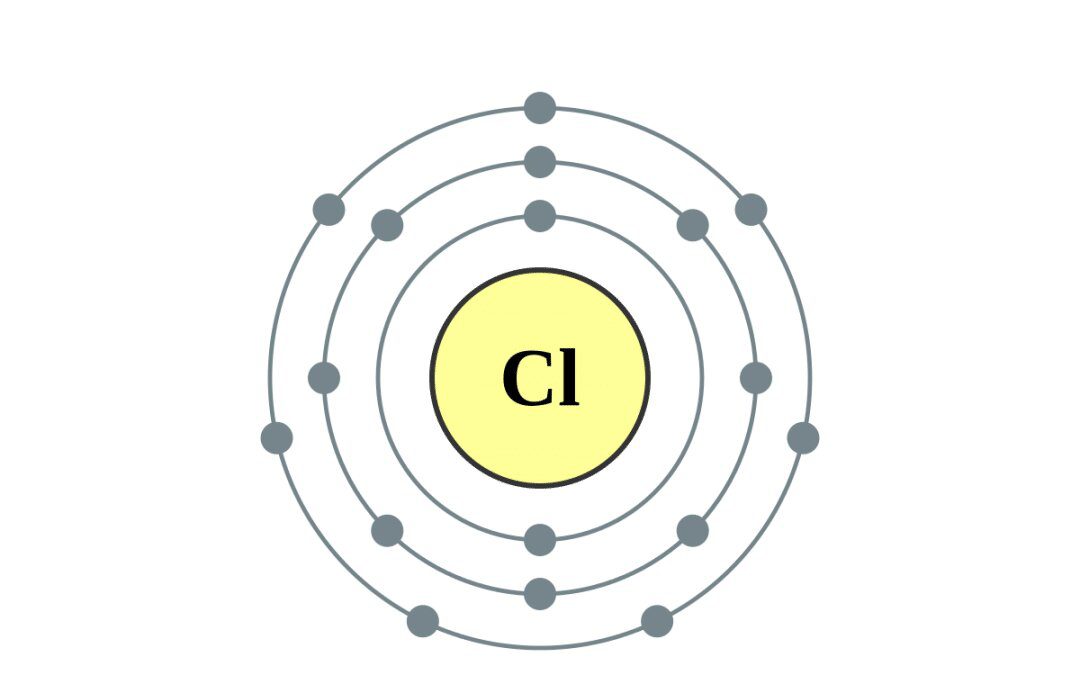
Chlorine is a chemical element with the atomic number 17 and the symbol Cl. It is the second-lightest of the halogens, occurring between fluorine and bromine on the periodic table, and its properties are often midway between them. Chlorine is a yellow-green gas at normal temperature. It is an extremely reactive element and a powerful oxidizing agent, with the highest electron affinity and third-highest electronegativity on the revised Pauling scale, behind only oxygen and fluorine. On electronegativity scales other than the revised Pauling scale, nitrogen is reported to be stronger than chlorine. These scales include the Allen, Allred-Rochow, Martynov-Batsanov, Mulliken-Jaffe, Nagle, and Noorizadeh-Shakerzadeh electronegativity scales.
Chlorine was widely used in medieval alchemists’ experiments, which frequently involved heating chloride salts like ammonium chloride (sal ammoniac) and sodium chloride (common salt), yielding various chlorine-containing chemical substances like hydrogen chloride, mercury(II) chloride (corrosive sublimate), and hydrochloric acid (in the form of aqua regia). However, about 1630, Jan Baptist van Helmont was the first to distinguish free chlorine gas as a unique chemical. In 1774, Carl Wilhelm Scheele reported chlorine gas, supposing it was an oxide of a new element. Chemists claimed in 1809 that the gas may be a pure element, which Sir Humphry Davy confirmed in 1810 by naming it after the Ancient Greek (khlrós, “pale green”) due to its hue.
All chlorine in the Earth’s crust, including table salt, exists as ionic chloride compounds due to its strong reactivity. It is the second most abundant halogen (after fluorine) in the Earth’s crust and the twenty-first most abundant chemical element. Regardless, the huge amounts of chloride in saltwater dwarf these crustal deposits.
Elemental chlorine is commercially produced from brine using electrolysis, especially in the chlor-alkali process. Because of elemental chlorine’s high oxidizing potential, it has been used to create commercial bleaches and disinfectants, as well as a reagent in a variety of chemical processes. Chlorine is used to make a wide range of consumer products, about two-thirds of which are organic compounds such as polyvinyl chloride (PVC), many intermediates for the manufacturing of plastics, and other end products that do not include the element. To maintain swimming pools clean, chlorine and chlorine-generating chemicals are used more directly. At high quantities, elemental chlorine is very poisonous and dangerous to most living things. During World War I, chlorine was used as a chemical warfare agent as a poison gas weapon.
All known forms of life require chlorine in the form of chloride ions. Other types of chlorine compounds are uncommon in live organisms, and chlorinated organics produced artificially range from harmless to hazardous. Organic substances containing chlorine, such as chlorofluorocarbons, have been related to ozone depletion in the upper atmosphere. Neutrophils create modest quantities of elemental chlorine as part of the immune system’s response to pathogens by oxidizing chloride ions.
https://pubs.acs.org/doi/10.1021/ed004p596
Chlorine is a reactive element that is intermediate in reactivity between fluorine and bromine. Chlorine is a lesser oxidizing agent than fluorine, although it is more powerful than bromine or iodine. The standard electrode potentials of the X2/X pairs show this (F, +2.866 V; Cl, +1.395 V; Br, +1.087 V; I, +0.615 V; At, about +0.3 V). This tendency, however, is not reflected in bond energies since fluorine is peculiar due to its tiny size, limited polarisability, and inability to exhibit hypervalence. Another distinction is that chlorine has substantial chemistry in positive oxidation states, but fluorine does not. Chlorination frequently produces greater oxidation states than bromination or iodination while producing lower oxidation states than fluorination. M-Cl bonds are formed when chlorine reacts with compounds containing M-M, M-H, or M-C bonds.
Given that E°(1/2 O2/H2O) = +1.229 V, which is less than +1.395 V, chlorine should be able to oxidize water to produce oxygen and hydrochloric acid. However, the kinetics of this reaction are unfavorable, and there is also a bubble overpotential effect to consider, thus electrolysis of aqueous chloride solutions produces chlorine gas rather than oxygen gas, which is highly helpful for commercial chlorine production.
http://eaavmadrid2011.es/why-is-oxyclozanide-important-in-medicine/








