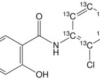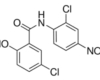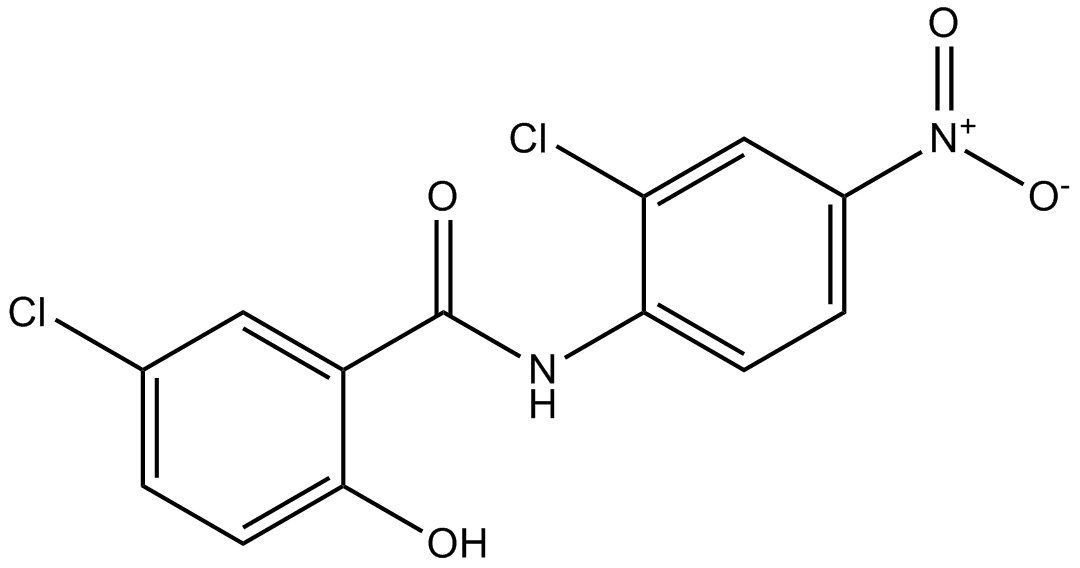
15 Mar Niclosamide and cancer
Adrenocortical carcinoma is a rare aggressive endocrine cancer for which surgical excision is the only effective but restricted treatment. Satoh et al. used a luciferase-coupled ATP quantification test to measure cell survival after screening a small molecule library containing 2492 medicines licensed for human use. Niclosamide was discovered to reduce adrenocortical carcinoma cellular proliferation, which was linked to apoptosis, epithelial-to-mesenchymal transition reduction, and -catenin levels. Furthermore, mitochondrial uncoupling activity was detected in cancer cells. Oral treatment of niclosamide inhibited tumor development with no harm detected.
Breast cancer and niclosamide
Breast cancer is the most common cause of mortality among women. To lower mortality, new medicines will need to be developed. According to Lu et al., niclosamide suppresses Wnt/-catenin signaling in breast cancer cells by boosting the degradation of the Wnt co-receptor LRP6. This group later showed that niclosamide, when combined with a monoclonal antibody that selectively activates TRAIL death receptor 5, inhibits tumor development in basal-like breast tumors. Niclosamide suppresses mTORC1 signaling in MCF-7 breast cancer cells, according to Fonseca et al. Mechanistic investigations revealed that Niclosamide decreased cytoplasmic pH and may indirectly inhibit mTORC1 signaling. Niclosamide has also been shown to inhibit non-breast cancer stem cells from transforming into cancer stem cells. This process is linked to the suppression of the IL6-JAK1-STAT3 signaling pathway. Using a cell-based STAT3-dependent dual luciferase reporter experiment, Ren et al. discovered niclosamide as a powerful STAT3 inhibitor capable of suppressing STAT3 transcriptional activity. Wang et al. employed a high-throughput drug screen to find that niclosamide prevented the development of breast cancer spheroid growth, caused apoptosis in vitro, and increased tumor growth in vivo. According to Karakas et al., niclosamide increased the anticancer activity of a palladium (II) saccharinate complex of terpyridine, resulting in increased cytotoxic activity in breast cancer stem cells.
Triple-negative breast cancer is distinguished by the absence of estrogen and progesterone receptor expression as well as HER2 amplification. It accounts for roughly 15% of all breast cancers and has no viable treatments. In vitro and in vivo, niclosamide suppresses ionizing radiation-induced Wnt/-catenin signaling in triple-negative breast cancer cells, according to Yin et al. According to Liu et al., niclosamide, either alone or in combination with cisplatin, inhibits the development of xenografts of cisplatin-resistant triple-negative breast cancer cells. They discovered that niclosamide reversed the epithelial-to-mesenchymal transition phenotype, inhibited the Akt, ERK, and Src signaling pathways, and decreased the proliferation of both CS and CR triple-negative breast cancer 231 cells in vitro. Niclosamide, either alone or in combination with cisplatin, has also been shown to inhibit the development of xenografts in mice carrying either 231-CS or 231-CR cells.
Colon cancer and niclosamide
In the United States, colorectal cancer is the second highest cause of cancer-related fatalities. Current chemotherapy regimens do not target the Wnt signaling system, which is one of the most critical underlying pathogenic processes. This pathway, which works via Wnt ligands interacting to cell surface Frizzled receptors to activate disheveled proteins that stabilize -catenin against constitutive destruction by the APC complex, has no FDA-approved drugs. This enables -catenin to concentrate and translocate to the nucleus, where it can influence gene transcription via the Tcf/Lef transcription factors. In a high throughput screen employing Frizzled1-GFP internalization from the cell surface, we found niclosamide as a small molecule inhibitor of Wnt/-catenin signaling from a small molecule library encompassing 1200 FDA-approved medicines. Niclosamide increased Wnt receptor Frizzled1 endocytosis, decreased Wnt3A-stimulated cytosolic -catenin stabilization, and inhibited Tcf/Lef gene reporter activity. Niclosamide suppressed the development of human colon cancer cells in vitro and in vivo, regardless of APC mutations.
S100A4 is a known Wnt receptor gene. In order to explore a chemical library for substances altering S100A4 gene transcription, Sack et al. developed an S100A4 promoter-driven luciferase reporter assay in human colorectal cancer HCT116 cells. They discovered that niclosamide inhibits S100A4 gene transcription. Niclosamide suppressed colon cell migration, invasion, proliferation, and colony formation in vitro, as well as liver metastasis in a mouse model. Suliman et al. investigated the effects of niclosamide on the proliferation and death of three colon cancer cell lines (HCT116, Lovo, and ad SW620). They discovered that niclosamide therapy was linked to Notch signaling pathway suppression and elevated expression of the tumor suppressor miR-200 family.
Glioma and niclosamide
Glioblastoma is the most prevalent primary brain tumor and has the highest fatality rate of all brain cancers. Current medicines are ineffective at lowering mortality. Wieland et al. used a cell viability test to examine a limited collection of 160 synthetic and natural chemicals and discovered that niclosamide decreased glioblastoma cell viability specifically. The Wnt, Notch, mTOR, and NF-B signaling pathways were all inhibited by niclosamide, according to detailed mechanistic investigations. In vivo, pre-exposure to niclosamide dramatically reduced the malignant potential of glioma cells.
Head and neck cancer and niclosamide
Head and neck cancers are a collection of malignancies that develop in the lip, oral cavity, nasal cavity, pharynx, larynx, and paranasal sinuses. Squamous cell carcinoma is the most frequent kind of head and neck cancer. In the United States, head and neck cancer accounts for roughly 3% of all cancer fatalities. According to Li et al., suppression of EGFR by erlotinib, an FDA-approved therapeutic drug, activated STAT3 signaling in head and neck cancer cells, which may be responsible for erlotinib’s lower therapeutic effectiveness against head and neck malignancies. The capacity of niclosamide to suppress STAT3 signaling resulted in in vitro and in vivo growth inhibition of head and neck cancer cells, as well as an increased anti-tumor impact of elrotinib.
Leukemia and niclosamide
Leukemias are malignancies of bone marrow stem cells or bone marrow-derived progenitors that result in an abnormally large number of white blood cells in circulation and blood-forming organs such as the bone marrow and lymphatic system. In the United States, around 50,000 cases of leukemia are diagnosed each year.
The Notch signaling system is essential for the development of hematopoietic stem cells. When Notch receptors are activated, they cleave, releasing the Notch intracellular domain (NICD), which travels to the nucleus and interacts to transcription factors like CBF1 to change gene expression. Wang et al. looked for small chemical modulators of CBF1-dependent Notch signaling using a CBF1-driven luciferase reporter system. They discovered that niclosamide inhibited endogenous Notch signaling in AML cells, a cell line generated from a patient with acute myelogenous leukemia. Jin et al. discovered that niclosamide suppresses TNF-induced NF-B-dependent reporter activity while increasing reactive oxygen species (ROS) levels in AML cells. In vitro, niclosamide synergized with the chemotherapeutic drugs cytarabine, etoposide, and daunorubicin, inhibiting the development of AML cells transplanted in nude mice. Recently, Jin et al. discovered that niclosamide reduces the long-term engraftment of chronic myelogenous leukemia (CML) CD34+ stem cells implanted in immunodeficient NOG mice and increases the survival of mice bearing leukemia cells driven by the human Bcr-Abl gene fusion, a common chromosomal translocation mutant driver of leukemia. The process might entail the interruption of a positive feedback loop between NF-B and FOXM1/-catenin, resulting in decreased self-renewal potential and CML survival.
Lung cancer and niclosamide
Lung cancer is the second most frequent cancer in the United States, but it has the highest fatality rate. Approximately 20% of individuals with non-small cell lung cancer (NSCLC) have EGFR gene mutations, which enhance cancer cell proliferation. EGFR inhibitors (for example, erlotinib) have been used, although drug resistance has evolved, reducing the efficacy of this class of medicines. Erlotinib resistance was linked to STAT3-Bcl2-Bcl-XL signaling, according to Li et al. Erlotinib resistance was overcome with niclosamide therapy. Niclosamide, in conjunction with erlotinib, inhibited the development of erlotinib-resistant lung cancer cell xenografts and promoted tumor apoptosis. The same group of researchers later showed that niclosamide is beneficial in lowering radio-resistance in human lung tumors both in vitro and in vivo. The process includes radiation-induced suppression of JAK2-STAT3 activity. Using a cell viability test, Lee et al. discovered that niclosamide increased radiosensitivity in the non-small cell lung cancer cell line H1299. This shows that niclosamide may be effective as a radiosensitizer in people with lung cancer.
Osteosarcoma and niclosamide
The most prevalent primary bone cancer is osteosarcoma. Despite breakthroughs in surgical procedures and chemotherapy regimens, patient survival in osteosarcoma has increased only little. According to Liao et al., niclosamide significantly inhibits osteosarcoma cell growth, migration, and survival. This inhibitory impact is linked to lower levels of c-Fos, c-Jun, E2F1, and c-Myc expression. Niclosamide also reduces the development of osteosarcoma tumors in a mouse xenograft tumor model.
Ovarian cancer and niclosamide
Ovarian cancer accounts for 3% of all female cancers. Yo et al. used a bioactive chemical library to screen for inhibitors of spheroid formation by cisplatin-resistant CP70 ovarian cancer cells, and found niclosamide to be an active medication in this test. Following that, niclosamide was discovered to inhibit ovarian tumor-initiating cells in vitro and in vivo through altering metabolic pathways.
Haygood et al. extracted ovarian cancer tumor spheres from patients and treated them with niclosamide (or analogs) and carboplatin simultaneously, seeing cytotoxicity. Wnt sensitive genes were suppressed in drug-treated samples,. Wnt7A and FGF1 expression are closely associated in ovarian carcinomas, according to King et al., and FGF1 is a direct transcriptional target of Wnt7A/-catenin signaling. Niclosamide inhibited Wnt7A/-catenin signaling, reducing -catenin transcriptional activity and cell survival while increasing cell death. In xenograft animal models, oral niclosamide suppressed tumor development and progression of human ovarian malignancies.
Prostate cancer and niclosamide
Prostate cancer is the most frequent cancer in males in the United States, and it is also the leading cause of cancer mortality in men. Because many prostate cancers are androgen-dependent, anti-androgens are effective treatments. Enzalutamide is a novel anti-androgen for the treatment of castration-resistant metastatic prostate cancer. Resistance to enzalutamide treatment has been linked to the development of androgen receptor splice variants such as the AR-V7 isoform. To find prospective medicines against the activity of the AR-V7 isoform, Liu et al. used an androgen-stimulated luciferase activity test. They discovered that niclosamide decreased AR-V7 protein expression, hindered AR-V7 transcription activity, and decreased AR-V7 recruitment to the prostate-specific antigen promoter. Niclosamide also decreased prostate cancer cell growth in vitro and tumor development in vivo, and it synergized with enzalutamide to limit the growth of enzalutamide-resistant tumors, indicating its potential use in enzalutamide-resistant patients. Following that, Liu et al. discovered that niclosamide reduced STAT3 activity in prostate cancer cells. Niclosamide restored enzalutamide resistance in prostate cancer cells synergistically, and niclosamide plus enzalutamide therapy resulted in colony formation suppression and growth arrest through causing cell death. The mechanism by which niclosamide overcomes enzalutamide resistance may be linked to the down-regulation of STAT3 target genes and the prevention of androgen receptor recruitment to the prostate-specific antigen promoter in prostate cancer cells in an IL6-dependent way. These findings imply that niclosamide, in order to overcome enzalutamide resistance and limit tumor cell migration and invasion in advanced prostate cancer, may target the IL6-STAT3-AR pathway.
Therapeutic treatments (including androgen deprivation therapy), chemotherapeutic drugs, and radiation have all been shown to cause neuroendocrine development in prostate cancer cells. These differentiated cells are resistant to therapies because they lack expression of the androgen receptor and prostate specific antigen. According to Ippolito et al., the ability of niclosamide to impede mitochondrial activity is linked to acidic pH in prostate neuroendocrine carcinoma cells. In a castration-resistant neuroendocrine prostate cancer cell line, niclosamide shows pH-dependent toxicity.
http://eaavmadrid2011.es/usage-and-dosage-instructions-for-niclosamide/
Renal cell cancer and niclosamide
Renal cell cancer develops from the renal tubule epithelium. It is the most frequent kind of kidney cancer in adults, and it is resistant to existing treatments, In two renal cell cancer cell lines, Zhao et al. reported that niclosamide suppresses proliferation and anchorage-independent colony formation. Niclosamide, in combination with cisplatin, inhibited tumor development in two in vivo renal cell carcinoma xenograft mice models via a mechanism involving both reduced -catenin expression and mitochondrial dysfunction.